Products
New Fashion Design for Pharmaceuticals Inc - China Diethyl Maleate Manufacture Supplier – Longo Detail:
Structural Formula
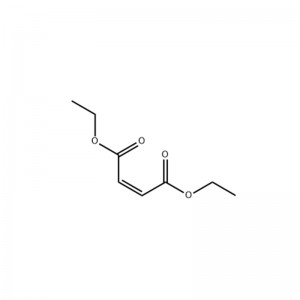
Physical Properties
Appearance: colorless transparent liquid
Density: 1.064 g/mL at 25 °C (lit.)
Melting point: -10 °C (lit.)
Boiling point: 225 °C (lit.)
Vapor density:5.93 (vs air)
Vapor pressure:1mm Hg (14°C)
Refractivity:n20/D 1.441 (lit.)
Flash Point: 200°F
Safety Data
It belongs to common goods
Customs code:2917190090
Export Tax Refund Rate(%):9%
Application
It is used as a pesticide intermediate for preparing malathion, an organophosphorus pesticide, and an intermediate for medicine, perfume and water quality stabilizer (organic polycarboxylic acid phosphonic acid compound). It can also be used as solvent for resin and nitrocellulose, plasticizer, organic synthesis, insecticide, polymer monomer and plastic assistant.
Properties and stability
Stable at room temperature and pressure. Prohibited substances: oxidizing agents, reducing agents, acids, bases. Can be burned, pay attention to the source of fire when using and storing. Prevent inhalation of vapors and avoid contact with skin.
Storage method
Store in a cool, dry and ventilated place. Pay attention to the source of fire when using and storing.
Synthesis method
1. It is produced by esterification of maleic anhydride and ethanol in the presence of sulfuric acid; it can also be obtained by exchange conversion with cation exchange resin as catalyst. The content of diethyl maleate in industrial product is ≥98%, and each ton of product consumes 585kg of maleic anhydride (95%) and 604kg of ethanol (95%).
2. Its preparation method is mainly made by esterification of maleic anhydride and ethanol in the presence of sulfuric acid. This process has two kinds of atmospheric pressure with benzene esterification and negative pressure without benzene esterification.
(1) Atmospheric pressure with benzene esterification
Add a certain amount of benzene and ethanol to the esterification reaction pot, put in maleic anhydride, add concentrated sulfuric acid dropwise under stirring, heat through jacketed steam, and make the reactants undergo esterification reaction at about 75℃. The generated water is removed by ternary azeotropic distillation with benzene and ethanol, and the upper layer of benzene and ethanol liquid is refluxed to the reaction pot. About 13 ~ 14h later, when the distillation tower temperature rises to 68.2 ℃, the separator lower water level is no longer rising, indicating that all the water in the reaction pot has been evaporated, the esterification reaction is complete. Stop reflux, continue distillation to 95-100 ℃, distillation of benzene and ethanol. Cool down to about 50℃, neutralize with 5% aqueous sodium carbonate solution, wash with water and then remove the residual benzene and ethanol under vacuum to obtain the product diethyl maleic acid.
(2) Negative pressure benzene-free esterification
Esterification of maleic anhydride and ethanol under the action of sulfuric acid is carried out under a certain vacuum and temperature to bring out the ethanol and the water generated by the reaction in a gaseous state, and then the ethanol is separated through the fractionation column to reflux the esterification, so that the reaction tends to be complete. This method can shorten the reaction cycle, improve the yield and product quality, improve the operating environment, most domestic production plants use this method.
In addition, cation exchange resin can also be used as a catalyst for exchange conversion to produce diethyl maleic acid.
Refining method: washing with dilute potassium carbonate solution, drying with anhydrous potassium carbonate or sodium sulfate and distillation under reduced pressure.
Product detail pictures:
Related Product Guide:
We can easily normally satisfy our respected buyers with our excellent high-quality, excellent selling price and good service due to we've been far more expert and more hard-working and do it in cost-effective way for New Fashion Design for Pharmaceuticals Inc - China Diethyl Maleate Manufacture Supplier – Longo , The product will supply to all over the world, such as: Australia, Uruguay, Buenos Aires, Insisting over the high-quality generation line management and prospects guide provider, we've made our resolution to offer our shoppers using the initially stage purchasing and soon after provider working experience. Preserving the prevailing helpful relations with our prospects, we even now innovate our product lists the many time to meet up with the brand new wants and stick to the latest trend of this business in Ahmedabad. We're ready to facial area the difficulties and make the transform to grasp many of the possibilities in international trade.
We have worked with many companies, but this time is the ,detailed explanation, timely delivery and quality qualified, nice!