Products
Chinese Professional Aztreonam Sodium Carbonate Mixed Powder - China Adenosine Diphosphate Manufacture Supplier – Longo Detail:
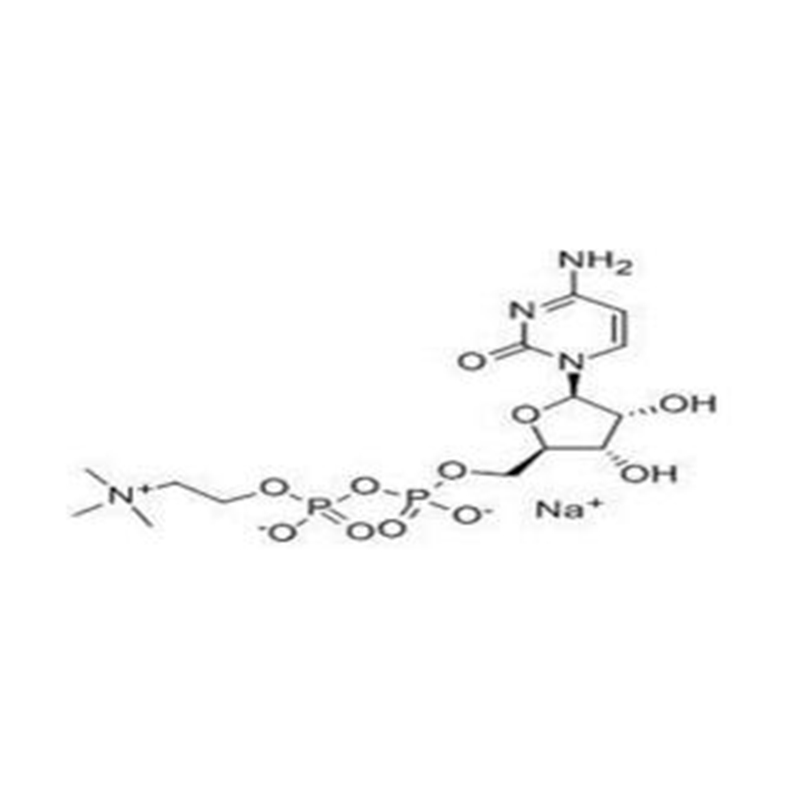
Structural Formula
Physical
Density: 2.49±0.1 g/cm3(Predicted)
Melting point.
Boiling point: 196°C
Refractivity
Flash point.
Chemical Properties
1.Stable at room temperature and pressure
2.Materials to avoid:Moisture/moisture Oxide
Safety Data
Hazardous category.
Dangerous Goods Transport Number.
Packing category.
Application
Adenosine diphosphate (ADP), also known as adenosine pyrophosphate (APP), is an important organic compound in metabolism and is essential to the flow of energy in living cells. ADP consists of three important structural components: a sugar backbone attached to adenine and two phosphate groups bonded to the 5 carbon atom of ribose. The diphosphate group of ADP is attached to the 5’ carbon of the sugar backbone, while the adenine attaches to the 1’ carbon.
ADP can be interconverted to adenosine triphosphate (ATP) and adenosine monophosphate (AMP). ATP contains one more phosphate group than does ADP. AMP contains one fewer phosphate group. Energy transfer used by all living things is a result of dephosphorylation of ATP by enzymes known as ATPases. The cleavage of a phosphate group from ATP results in the coupling of energy to metabolic reactions and a by-product of ADP.[1] ATP is continually reformed from lower-energy species ADP and AMP. The biosynthesis of ATP is achieved throughout processes such as substrate-level phosphorylation, oxidative phosphorylation, and photophosphorylation, all of which facilitate the addition of a phosphate group to ADP.
Adenosine diphosphate (also called adenosine diphosphate) is a compound consisting of a molecule of adenosine with two attached phosphate roots, its molecular formula is C10H15N5O10P2. In living organisms, it is usually the product of the hydrolysis of adenosine triphosphate (ATP) after the loss of a phosphate root, i.e., the breaking of a high-energy phosphate bond, and the release of energy.
Product detail pictures:
Related Product Guide:
It is our responsibility to meet your needs and efficiently serve you. Your satisfaction is our satisfactory reward. We are looking forward to your visit for joint growth for Chinese Professional Aztreonam Sodium Carbonate Mixed Powder - China Adenosine Diphosphate Manufacture Supplier – Longo , The product will supply to all over the world, such as: Benin, Hamburg, Libya, Being guided by customer demands, aiming at improving the efficiency and quality of customer service, we constantly improve products and provide more comprehensive services. We sincerely welcome friends to negotiate business and start cooperation with us. We hope to join hands with friends in different industries to create a brilliant future.
The sales manager is very patient, we communicated about three days before we decided to cooperate, finally, we are very satisfied with this cooperation!