Products
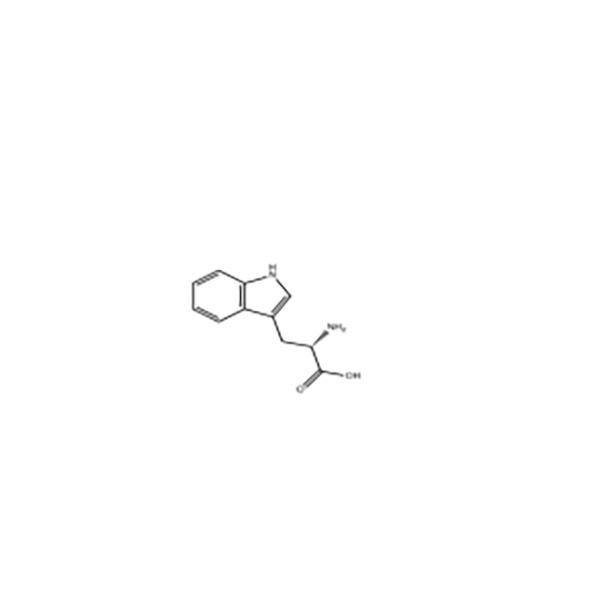
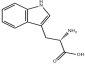
L-Tryptophan
Structural Formula
Appearance:white Crystal Powder
Density:1.34
Melting Point:289-290 °c (dec.)(lit.)
Boiling Point:342.72°c (rough Estimate)
Refractivity:-32 °(c=1, H2o)
Solubility :20% Nh3: 0.1 g/ml At 20 °c, Clear, Colorless
Ph:5.5-7.0 (10g/l, H2o, 20℃)
Solubility In Water: 11.4 G/l (25 ºc)
Spinability:[α]20/d 31.5±1°, C = 1% In H2O
Safety Data
Hazard category:Not dangerous goods
Dangerous goods transport no:
Packaging category:
Application
1.Amino acid drugs. Uesd for improve depression with with iron and vitamins.As an insomnia sedative with L-dopa for the treatment of Parkinson's disease.
2.Nutritional supplements
3.Used in biochemical research, as a sedative in medicine
Character
Coloured by prolonged light. Coheating with water produces small amounts of indole. If heated in the presence of sodium hydroxide and copper sulphate, it produces large amounts of indole. Tryptophan is more stable when heated in the dark with acid. Very easy to decompose when coexisting with other amino acids, sugars and aldehydes. If no hydrocarbons are present, it remains stable when heated with 5 mol/L sodium hydroxide to 125°C. When proteins are decomposed with acid, tryptophan is completely decomposed, producing a putrid black substance.
When proteins are decomposed with acid, tryptophan is completely decomposed, producing a blackened substance. Tryptophan is a heterocyclic amino acid and an essential amino acid. In the body, it is converted into a variety of physiologically active substances such as 5-hydroxytryptamine, niacin, melanotropic hormone, pineal hormone and xanthurenic acid. When the body is deficient in tryptophan, it will not only cause general hypoproteinism, but also special diseases such as skin disorders, cataracts, vitreous degeneration and myocardial fibrosis. It also enhances the body's resistance to gamma radiation. The minimum daily requirement for humans is 0.2g.