Products
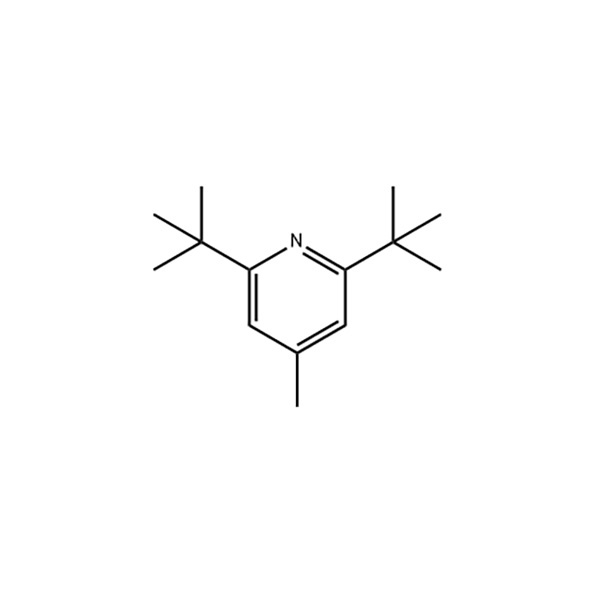
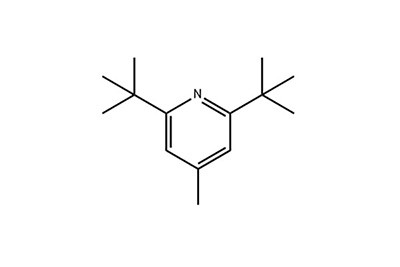
2,6-Di-tert-butyl-4-methylpyridine
Structural Formula
Appearance: white acicular crystal
Density: 1,476 g/cm3
Melting point: 31-32 ℃
Boiling point: 148-153℃ (12.6kPa)
Refractive index: N20 /D 1.4763(lit.)
Flash point: 183 °F
Storage conditions: 2-8°C
Acidity coefficient (pKa): 6.88±0.10(Predicted)
Safety Data
Belongs to general cargo
Customs code: 2942000000
Export tax rebate rate (%) : 13%
Application
Organic synthetic intermediate, a sterically hindered, non-nucleophilic base which distinguishes between Brnsted (protonic) and Lewis acids. Enables the direct high-yield conversion of aldehydes1 and ketones1,2 to vinyl triflates.
2,6-Di-tert-butyl-4-methylpyridine is an organic compound with molecular formula C14H23N, an important intermediate in organic synthesis, mainly used in pharmaceutical intermediates, organic synthesis, organic solvents, also can be applied in dye production, pesticide production and fragrances, etc.
Production method
1. Make 2,6 di-tert-butyl-4-methylbenzyl trifluoromethanesulfonate In a 100-mL triple flask equipped with a nitrogen introduction tube, a constant pressure funnel, an electromagnetic stirring bar and an ice condenser with a dry tube, add 24.2 g (0.2 mol) of trimethylethyl phthalide chloride and 3.7 g (0.05 mol) of tert-butanol. The reaction mixture was heated in an oil bath to 85°C. Then 15 g (0.1 mol) of trifluoromethanesulfonic acid was added for more than 15 minutes. The reaction was continued for 10 minutes, and the light brown reaction product was cooled in an ice bath and poured into 100 ml of cold ether. The brown precipitate was filtered and dried to obtain 9.6 g (54%) of the delayed buzz salt. (No purification is required: for the next step of preparation, recrystallize twice from chloroform to carbon tetrachloride 3:1) to colorless needle-like crystals.
2. Preparation of 2,6-Di-tert-butyl-4-methyl-vizine Under stirring, a suspension of 10 g (0.028 mol) of the crude salt of vizine in 200 mL of 95% ethanol chilled to 60°C was added once to 100 mL of concentrated ammonia chilled to 60°C in a 1-liter garden-bottom flask. The reaction was continued at 60°C for 30 minutes and 40°C for 2 hours, during which time the suspension was dissolved. Then, the reaction mixture was poured into 500 ml of 2% sodium hydroxide solution slowly warmed to room temperature. Extracted with .4-100 ml of pentane, combined extracts, 25 ml of saturated sodium chloride solution washed, 100 ml of pentane rotary evaporator concentration.